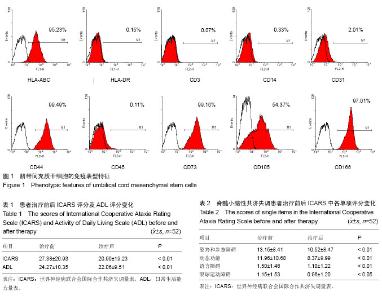
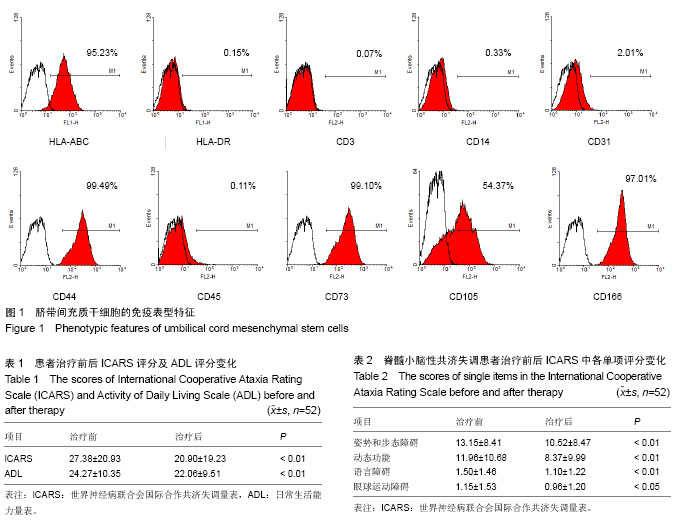
| [1] Messerli M, Wagner A, Sager R,et al.Stem cells from umbilical cord Wharton's jelly from preterm birth have neuroglial differentiation potential.Reprod Sci. 2013;20(12):1455- 1464.
[2] Momin EN, Mohyeldin A, Zaidi HA,et al.Mesenchymal stem cells: new approaches for the treatment of neurological diseases.Curr Stem Cell Res Ther. 2010;5(4):326-344.
[3] Zhang MJ, Sun JJ, Qian L,et al.Human umbilical mesenchymal stem cells enhance the expression of neurotrophic factors and protect ataxic mice.Brain Res. 2011; 1402:122-131.
[4] Avanzini MA, Bernardo ME, Cometa AM,et al.Generation of mesenchymal stromal cells in the presence of platelet lysate: a phenotypic and functional comparison of umbilical cord blood- and bone marrow-derived progenitors.Haematologica. 2009;94(12):1649-1660.
[5] 黄红云.中枢神经修复学[M].北京:科学出版社,2009:P479-482.
[6] 日常生活能力量表(ADL)[J].中国微侵袭神经外科杂志,2006, 11(11):516.
[7] 崔海燕,李旭光,朱敏霞,等.脊髓小脑性共济失调的研究进展[J].医学综述,2011,17(19):2958-2960.
[8] 刘钰鹏.脊髓小脑性共济失调的基因研究进展[J].医学综述,2013, 19(15):2722-2725.
[9] Shetty P, Cooper K, Viswanathan C.Comparison of proliferative and multilineage differentiation potentials of cord matrix, cord blood, and bone marrow mesenchymal stem cells.Asian J Transfus Sci. 2010;4(1):14-24.
[10] Dalous J, Larghero J, Baud O.Transplantation of umbilical cord-derived mesenchymal stem cells as a novel strategy to protect the central nervous system: technical aspects, preclinical studies, and clinical perspectives.Pediatr Res. 2012; 71(4 Pt 2):482-490.
[11] Taghizadeh RR, Cetrulo KJ, Cetrulo CL.Wharton's Jelly stem cells: future clinical applications.Placenta. 2011;32 Suppl 4: S311-315.
[12] Fan CG, Zhang QJ, Zhou JR.Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev. 2011;7(1):195-207.
[13] Soleymaninejadian E, Pramanik K, Samadian E.Immunomodulatory properties of mesenchymal stem cells: cytokines and factors.Am J Reprod Immunol. 2012;67(1):1-8.
[14] Cho PS, Messina DJ, Hirsh EL,et al.Immunogenicity of umbilical cord tissue derived cells.Blood. 2008;111(1): 430-438.
[15] Lin YC, Ko TL, Shih YH,et al.Human umbilical mesenchymal stem cells promote recovery after ischemic stroke.Stroke. 2011;42(7):2045-2053.
[16] Lim JY, Jeong CH, Jun JA,et al.Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells after intrathecal administration by lumbar puncture in a rat model of cerebral ischemia.Stem Cell Res Ther. 2011;2(5):38.
[17] Chang YK, Chen MH, Chiang YH,et al.Mesenchymal stem cell transplantation ameliorates motor function deterioration of spinocerebellar ataxia by rescuing cerebellar Purkinje cells.J Biomed Sci. 2011;18:54.
[18] Jones J, Jaramillo-Merchán J, Bueno C,et al.Mesenchymal stem cells rescue Purkinje cells and improve motor functions in a mouse model of cerebellar ataxia.Neurobiol Dis. 2010; 40(2):415-423.
[19] Dey R, Kemp K, Gray E,et al.Human mesenchymal stem cells increase anti-oxidant defences in cells derived from patients with Friedreich's ataxia.Cerebellum. 2012;11(4):861-871.
[20] Kemp K, Mallam E, Hares K,et al.Mesenchymal stem cells restore frataxin expression and increase hydrogen peroxide scavenging enzymes in Friedreich ataxia fibroblasts.PLoS One. 2011;6(10):e26098.
[21] Park HJ, Bang G, Lee BR,et al.Neuroprotective effect of human mesenchymal stem cells in an animal model of double toxin-induced multiple system atrophy parkinsonism.Cell Transplant. 2011;20(6):827-835.
[22] Glavaski-Joksimovic A, Bohn MC.Mesenchymal stem cells and neuroregeneration in Parkinson's disease.Exp Neurol. 2013;247:25-38.
[23] Weiss ML, Medicetty S, Bledsoe AR,et al.Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24(3):781-792.
[24] Lee PH, Lee JE, Kim HS,et al.A randomized trial of mesenchymal stem cells in multiple system atrophy.Ann Neurol. 2012;72(1):32-40.
[25] Jin JL, Liu Z, Lu ZJ,et al.Safety and efficacy of umbilical cord mesenchymal stem cell therapy in hereditary spinocerebellar ataxia.Curr Neurovasc Res. 2013;10(1):11-20.
[26] Dongmei H, Jing L, Mei X,et al.Clinical analysis of the treatment of spinocerebellar ataxia and multiple system atrophy-cerebellar type with umbilical cord mesenchymal stromal cells.Cytotherapy. 2011;13(8):913-917.
[27] Paul G, Anisimov SV.The secretome of mesenchymal stem cells: potential implications for neuroregeneration.Biochimie. 2013;95(12):2246-2256.
[28] Satake K, Lou J, Lenke LG.Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue.Spine (Phila Pa 1976). 2004;29(18):1971-1979.
[29] El Omar R, Beroud J, Stoltz JF,et al.Umbilical Cord Mesenchymal Stem Cells: The New Gold Standard for Mesenchymal Stem Cell-Based Therapies?Tissue Eng Part B Rev. 2014 Apr 22. [Epub ahead of print]
[30] Stemberger S, Jamnig A, Stefanova N,et al.Mesenchymal stem cells in a transgenic mouse model of multiple system atrophy: immunomodulation and neuroprotection.PLoS One. 2011;6(5):e19808.
[31] Liu J, Han D, Wang Z,et al. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells.Cytotherapy. 2013;15(2):185-191.
[32] Sun T, Ma QH.Repairing neural injuries using human umbilical cord blood.Mol Neurobiol. 2013;47(3):938-945. |